OneClass: Predict the structures of BOTH bronsted acid base reaction of NaH with typical thiol. What ...
✓ Solved: When 4-chlorobutane-1-thiol is treated with a strong base such as sodium hydride, NaH, tetrahydrothiophene...

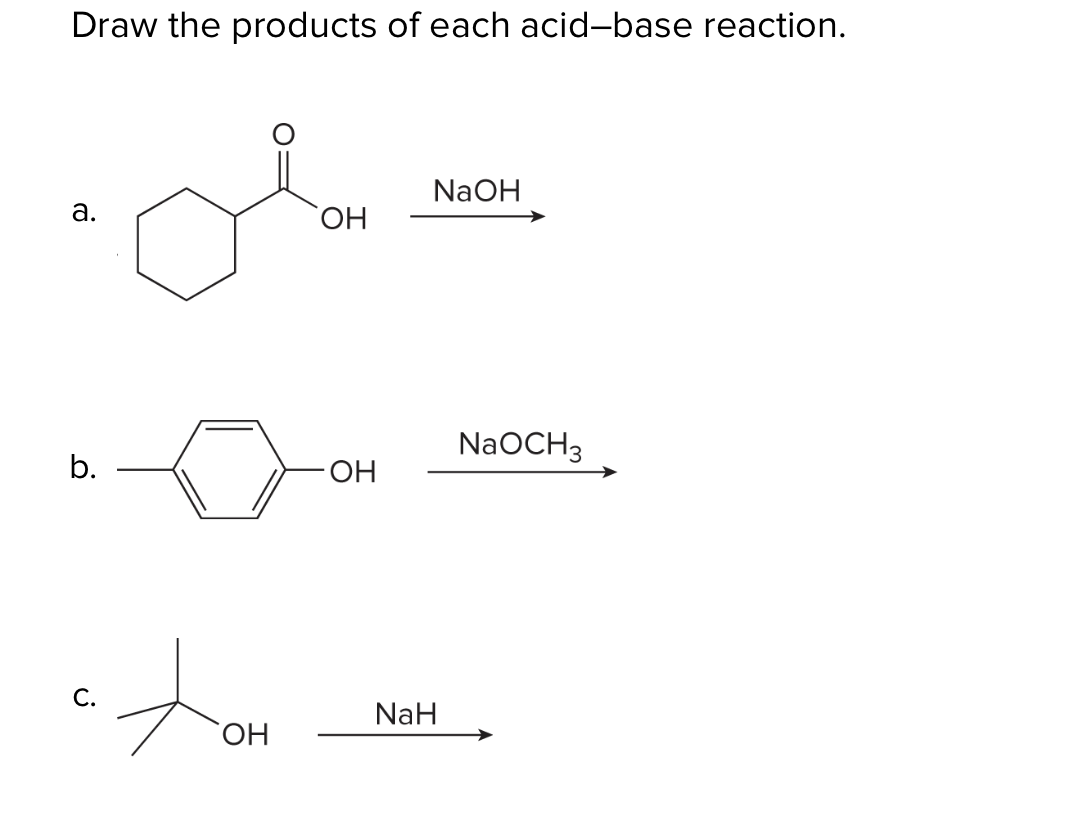
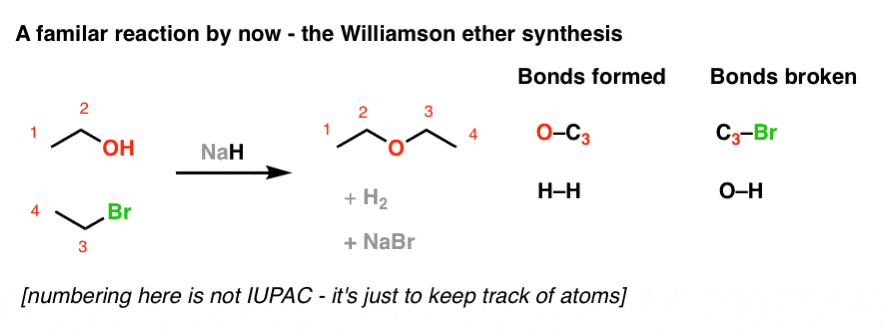
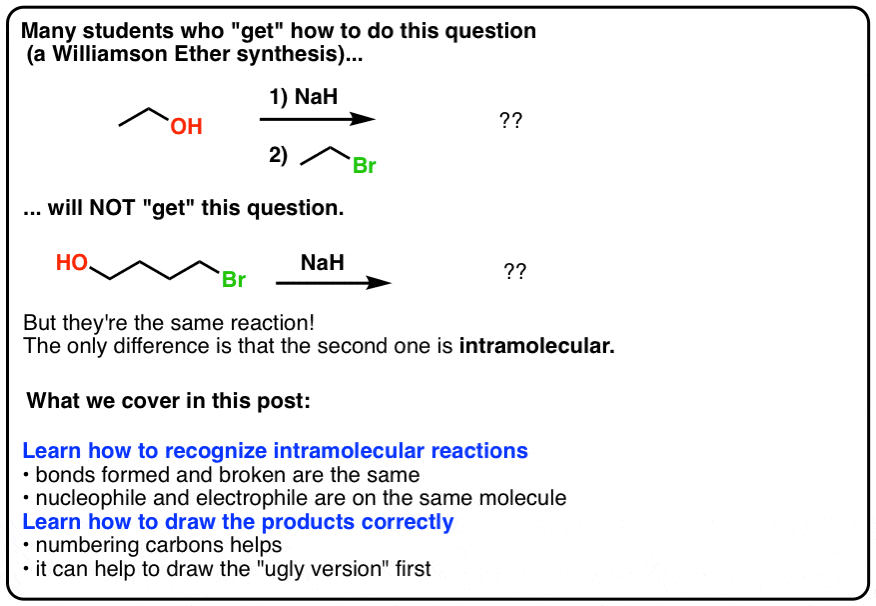
What product is formed when the given compound is treated with NaH? The given acid-base reactions were a step in a synthesis of a commercially available drug. | Homework.Study.com

OneClass: Predict the structures of BOTH bronsted acid base reaction of NaH with typical thiol. What ...

SOLVED: Write out the acid-base reaction of propyne (CH) with sodium hydride (NaH) This mcans draw out the reactants and products Provide curved arrows to show the electron flow (5 pts) Put

The hydride ion ( H^ - ) is stronger base than OH^ - ion. Which of the following reaction will occurs if sodium hydride (NaH) is dissolved in water?

Complications from dual roles of sodium hydride as a base and as a reducing agent. - Abstract - Europe PMC

The Employment of Sodium Hydride as a Michael Donor in Palladium‐catalyzed Reductions of α, β‐Unsaturated Carbonyl Compounds - Liu - 2019 - Advanced Synthesis & Catalysis - Wiley Online Library

The hydride ion ( H^ - ) is stronger base than OH^ - ion. Which of the following reaction will occurs if sodium hydride (NaH) is dissolved in water?