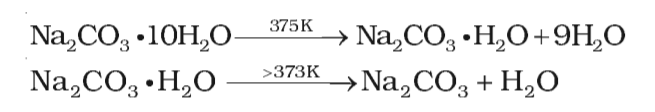
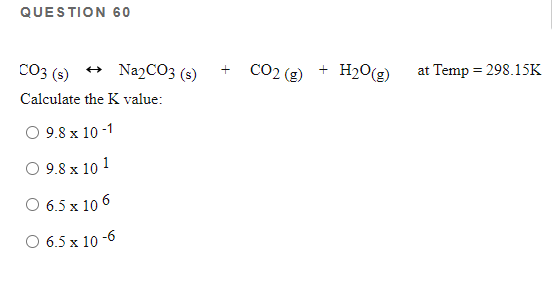Preparation and thermal properties of Na2CO3·10H2O-Na2HPO4·12H2O eutectic hydrate salt as a novel phase change material for energy storage - ScienceDirect

Washing soda has the formula Na 2 CO 3· 10 H 2 O. What mass of anhydrous sodium carbonate is left when all the water of crystallization is expelled by heating 57.2

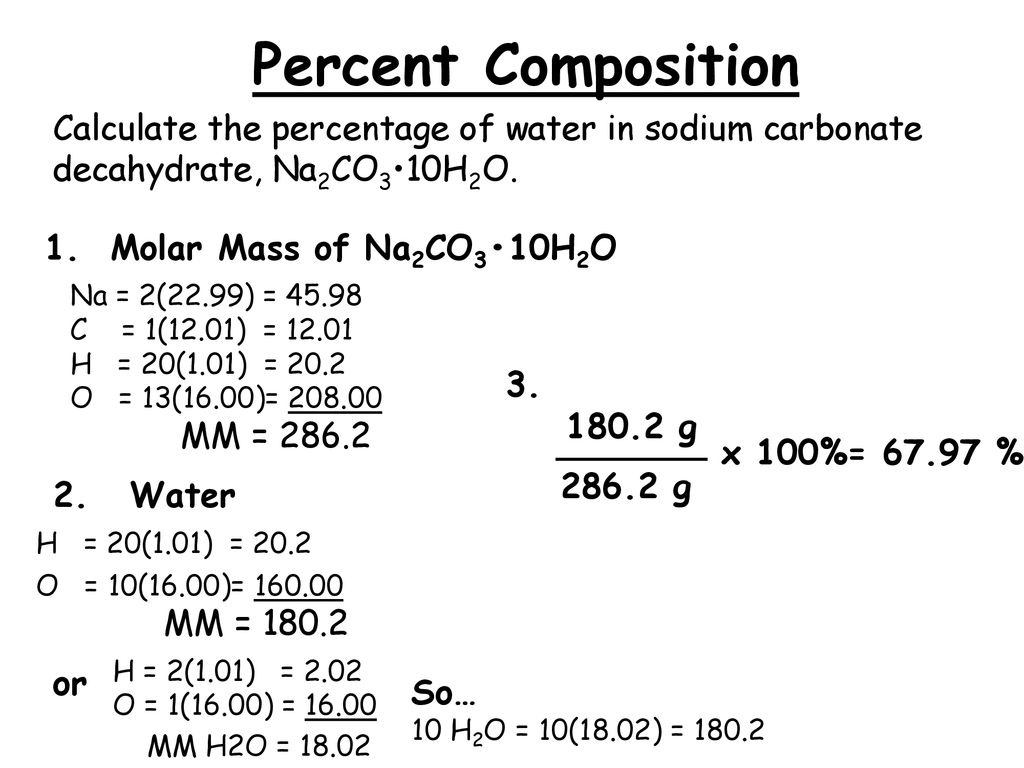
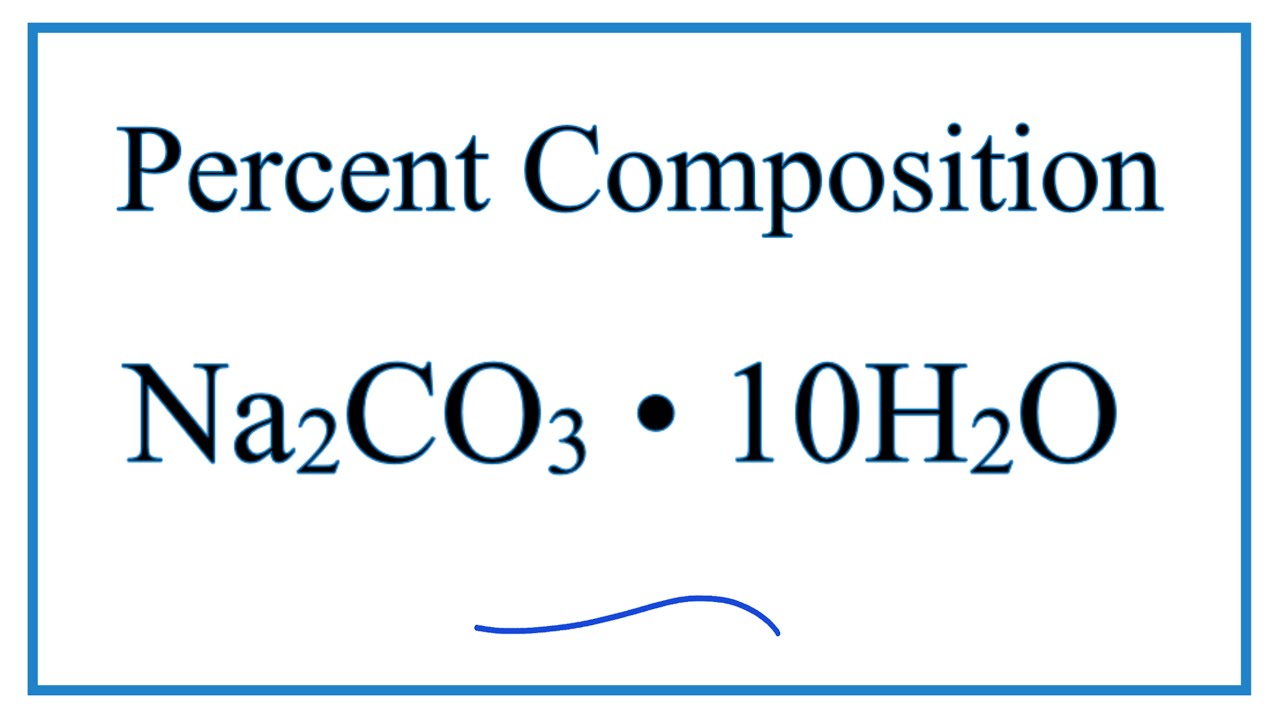
SOLVED: What is the percentage of water in the following compound? Sodium carbonate decahydrate, Na2CO3 • 10H2O % by mass H2O
Washing soda has the formula Na2CO3 .10H2O. What mass of anhydrous sodium carbonate is - Sarthaks eConnect | Largest Online Education Community

Calculate the formula mass of sodium carbonate (Na2CO3.10H2O) - CBSE Class 9 Science - Learn CBSE Forum